Hydrophosphination-type reactivity promoted by bismuth phosphanides: scope and limitations†
Abstract
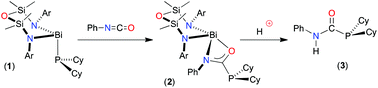
Phenyl isocyanate inserts into the Bi–P bond of the terminal phosphanide Bi(NONAr)(PCy2) (NONAr = [O(SiMe2NAr)2]2− Ar = 2,6-iPr2C6H3) to afford the κ2N,O-phosphanylcarboxamidate complex. Liberation of the hydrophosphination product from the metal is achieved by reaction with HPPh2. The diphenylphosphanide product, Bi(NONAr)(PPh2), is however unstable and decomposes to generate reduced species, preventing catalytic turnover.


 Please wait while we load your content...
Please wait while we load your content...