N-Heterocyclic carbene stabilized parent sulfenyl, selenenyl, and tellurenyl cations (XH+, X = S, Se, Te)†
Abstract
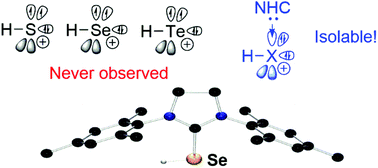
NHC-stabilized parent sulfenyl (H–S+), selenenyl (H–Se+) and tellurenyl (H–Te+) cations have been achieved by treatment of NHC chalcogen adducts with trifluoromethanesulfonic acid. Computational investigations show that most of the positive charges are localized at chalcogen atoms and carbene carbons with strong donor acceptor interactions between the lone pair of chalcogen atoms and the vacant orbital of the carbene centre, accounting for its unexpected stability.


 Please wait while we load your content...
Please wait while we load your content...