Composition dependent magnetic and ferroelectric properties of hydrothermally synthesized GdFe1−xCrxO3 (0.1 ≤ x ≤ 0.9) perovskites
Abstract
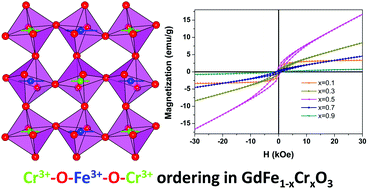
The hydrothermal synthesis and magnetic, dielectric and ferroelectric property characterization of ABO3-perovskite GdFe1−xCrxO3 (0 < x < 1) are reported. The mineralizer KOH plays a critical role in the perovskite structure of the sample. The Fe/Cr ratio of the final crystal is controlled by the alkalinity in the initial reaction mixtures. The lattice parameters and magnetic ordering transition temperature have a close relationship with the Fe/Cr ratio. The temperature dependent magnetic properties of GdFe1−xCrxO3 (0 < x < 1) samples show a close relationship with substituent ratios of Cr3+. The occurrence of ferromagnetic behavior in all of the as-prepared samples comes from the coexistence of canted-antiferromagnetism due to the double exchange effect of Fe–O–Fe and Cr–O–Cr and ferromagnetism due to superexchange interaction of Fe–O–Cr. A large spontaneous polarization was observed in GdFe1−xCrxO3 at room temperature, exhibiting a clear ferroelectric hysteresis loop, which indicates the ferroelectric behavior in this system. This work not only provides an effective route to the controllable synthesis of B-site doped REFe1−xCrxO3 perovskite structure materials, but also presents a comprehensive study of the tunable effect of ionic doping on ferroelectric properties.


 Please wait while we load your content...
Please wait while we load your content...