5-(2-Pyridil)-1H-tetrazole complexes with Mo(iv) and W(iv) cyanides†
Abstract
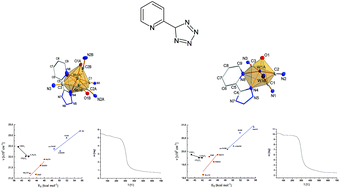
The reactions of tetrazole ligand derived from 2-cyanopyridine with K3Na[Mo(CN)4O2]·6H2O and K3Na[W(CN)4O2]·6H2O in water–ethanol solution result in isolation of two new complexes with the following formulae: (PPh4)2[Mo(CN)3O(pdt)]·2H2O (1) (Hpdt = 5-(2-pyridil)-1H-tetrazole) and (PPh4)2[W(CN)3O(pdt)]·3H2O (2). The complexes were characterized by elemental analysis, single crystal X-ray structure measurements, IR and UV-Vis spectroscopy, cyclic voltammetry measurement as well as thermogravimetric measurement. The X-ray crystal structure measurements indicated the presence of two coordination isomers in a single crystal. The pyridine nitrogen occupies both trans and/or cis position to the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, which is explained by similar pK of pyridine and tetrazole nitrogen. The DTG measurements indicate the stabilization of tetrazole upon coordination.
O bond, which is explained by similar pK of pyridine and tetrazole nitrogen. The DTG measurements indicate the stabilization of tetrazole upon coordination.


 Please wait while we load your content...
Please wait while we load your content...