Pseudocapacitive performance of a solution-processed β-Co(OH)2 electrode monitored through its surface morphology and area
Abstract
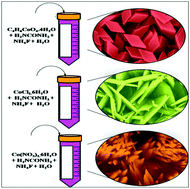
In the present study, beta-cobalt hydroxide (β-Co(OH)2) electrodes of various nanostructures and surface areas, viz. nano-rhombuses (NRs), nano-plates (NPs), and nano-grass (NGs), have been synthesized directly onto a stainless-steel (SS) substrate using a simple, economical and binder-free chemical solution-process, utilizing three cobalt precursor salts, i.e. cobalt acetate, cobalt chloride, and cobalt nitrate, respectively. Structural elucidation proves the crystallite size, type and phase-purity of β-Co(OH)2, whereas the surface morphology analysis supports the evolution of the above mentioned nanostructures of various surface areas. The electrochemical pseudocapacitor performance investigation demonstrates a specific capacitance (Sc) of 367 F g−1 at 1 mA cm−2 for the NP-type morphology, which is higher than that that displayed by the other morphologies. This change in Sc value is attributed to different charge transfer resistance values, which have been obtained from electrochemical impedance spectroscopy spectra. Finally, we attempt to correlate the relationship between the surface morphology, i.e. surface area, and the charge transfer resistance with the obtained specific capacitance value of the respective electrode.


 Please wait while we load your content...
Please wait while we load your content...