Group 12 metal complexes of (2-piperazine-1-yl-ethyl)-pyridin-2-yl-methylene-amine: rare participation of terminal piperazine N in coordination leads to structural diversity†
Abstract
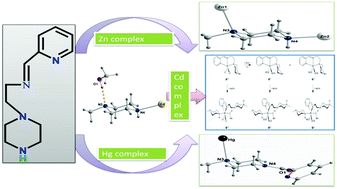
By using a potential tridentate ligand L ((2-piperazine-1-yl-ethyl)-pyridin-2-yl-methylene-amine), a series of group 12 metal complexes namely, [ZnLHCl2][Zn2LCl5]·2H2O (1), [CdL(SCN)2(CH3OH)]n (2), and [Hg(L-pyCO)Cl2] (3), were synthesized and structurally characterized. In all the complexes the piperazine nitrogen of the ligand takes part in coordination and leads to the complexes of group 12 metal ions having structural diversity. The X-ray diffraction analysis of complex 1 indicates for one Zn(II) ion a geometry in between trigonal bipyramidal/square pyramidal and for the second a distorted tetrahedral sphere. In the polymeric complex 2 the Cd(II) ion shows a distorted octahedral environment, while in the mononuclear complex 3, where Hg(II) exhibits a square-pyramidal geometry, an unexpected condensation between the uncoordinated NH piperazine fragment with 2-pyridinecarboxaldehyde was detected. The M–N bond lengths in all the complexes are in accordance with the metal ionic radius. Continuous shape measures through a DFT approach provide the coordination environment around each metal centre that is comparable with the experimental observations. We have also investigated the importance of hydrogen bonding of methanol in the generation of the polymeric Cd complex 2 along with the rearrangement of the tridentate ligand to generate an octahedral complex. The photoluminescence properties of the complexes as well as of the ligand were investigated in solution at ambient temperature. The low quantum yield of the ligand was ascribed due to a very fast photoinduced electron transfer (PET) from the nitrogen lone pair to the conjugated pyridine moiety. Complexation prevents the electron transfer, and consequently an increase in quantum yield was observed in the complexes. Among the three complexes the highest photoluminescence was exhibited by a Zn complex, being lower in Cd and Hg complexes as a consequence of the heavy atom perturbation effect.


 Please wait while we load your content...
Please wait while we load your content...