Electrodeposition of zinc nanoplates from an ionic liquid composed of 1-butylpyrrolidine and ZnCl2: electrochemical, in situ AFM and spectroscopic studies†
Abstract
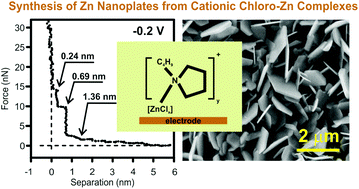
The mixtures of 1-butylpyrrolidine and ZnCl2 result in the formation of an ionic liquid, which can be used as an electrolyte for zinc electrodeposition. The feasibility of electrodepositing Zn from these electrolytes was investigated at RT and at 60 °C. The synthesized mixtures are rather viscous. Toluene was added to the mixtures to decrease the viscosity of the ILs. Vibrational spectroscopy was employed for the characterization of the liquids. The electrochemical behaviour of the liquids was evaluated by cyclic voltammetry. The electrode/electrolyte interface of this IL was probed by Atomic Force Microscopy (AFM). The suitable range for the electrodeposition of Zn was found to be ≥28.6 mol% of ZnCl2. Zn deposition occurs mainly from the cationic species of [ZnClxLy]+ (where x = 1, y = 1–2, and L = 1-butylpyrrolidine) in these electrolytes. This is in contrary to the well investigated chlorozincate ionic liquids where the deposition of Zn occurs mainly from anionic chlorozincates. Nanoplates of Zn were obtained from these mixtures of 1-butylpyrrolidine and ZnCl2.

 Please wait while we load your content...
Please wait while we load your content...