Replacement of quinolines with isoquinolines affords target metal ion switching from Zn2+ to Cd2+ in the fluorescent sensor TQLN (N,N,N′,N′-tetrakis(2-quinolylmethyl)-2,6-bis(aminomethyl)pyridine)†
Abstract
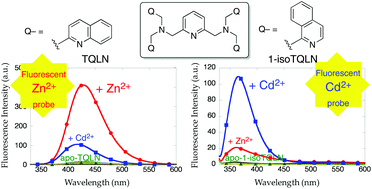
A quinoline-based heptadentate ligand, N,N,N′,N′-tetrakis(2-quinolylmethyl)-2,6-bis(aminomethyl)pyridine (TQLN), exhibits a Zn2+-specific fluorescence increase at 428 nm, which is assigned to excimer emission (IZn/I0 = 38, ICd/IZn = 24%, ϕZn = 0.069). In contrast, the isoquinoline counterpart 1-isoTQLN exhibits a Cd2+-specific fluorescence increase at 365 nm attributable to monomer emission (ICd/I0 = 83, IZn/ICd = 19%, ϕCd = 0.015).


 Please wait while we load your content...
Please wait while we load your content...