Oxygen vacancy ordering in SrFe0.25Co0.75O2.63 perovskite material†
Abstract
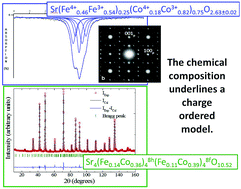
SrFe0.25Co0.75O2.63 was synthesized by a solid-state reaction. Its structural study at room temperature using conventional X-ray as well as neutron powder diffraction, electron diffraction and high-resolution transmission electron microscopy is presented. An oxygen-vacancy ordering related to the “314” model known for the Sr3Y1Co4O10.5 oxide is proposed despite neither an A-site ordering nor an A-site mismatch. By means of Mössbauer spectroscopy, Mohr salt titration and the difference in the neutron cross sections of Fe and Co, a cation distribution within the crystallographic sites as the following Sr4(Fe0.143+Co0.363+)48h(Fe0.114+Co0.144+Co0.253+)48fO10.52 is suggested, highlighting a natural layered structure with Fe and Co in higher oxidation states in the oxygen replete layers than in the oxygen deficient ones.

 Please wait while we load your content...
Please wait while we load your content...