Diversity of reactivity modes upon interplay between Au(iii)-bound isocyanides and cyclic nitrones: a theoretical consideration†‡
Abstract
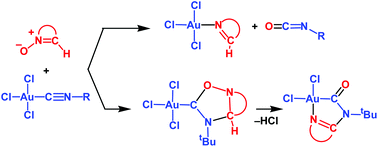
Metal-bound isocyanides demonstrate a very rich chemistry towards 1,3-dipoles of the allyl anion type such as nitrones. Depending on the metal centre, dipole nature and substituent in isocyanides, cycloadducts, imine + isocyanates or metallacycles may be formed. In this work, the reasons for such diversity, intimate details of the reaction mechanism and main factors determining the chemoselectivity in the reaction between Au(III)-bound isocyanides ([AuCl3(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NR)]) and cyclic nitrones −O–
NR)]) and cyclic nitrones −O–![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) +
+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)XX
C(H)XX![[X with combining low line]](https://www.rsc.org/images/entities/char_0058_0332.gif) (XXX = CH2CH2CMe2, CH2CH2CH2, OCH2CMe2, CH2OCMe2 and CH2CH2O) are analysed in detail by theoretical (DFT) methods. The formation of cycloadducts is controlled by the LUMOπ*(C
(XXX = CH2CH2CMe2, CH2CH2CH2, OCH2CMe2, CH2OCMe2 and CH2CH2O) are analysed in detail by theoretical (DFT) methods. The formation of cycloadducts is controlled by the LUMOπ*(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) of isocyanides and it occurs in a stepwise manner via the initial nucleophilic addition of nitrone at the C atom of C
N) of isocyanides and it occurs in a stepwise manner via the initial nucleophilic addition of nitrone at the C atom of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NR (except for nitronate −O–
NR (except for nitronate −O–![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) +
+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)CH2CH2
C(H)CH2CH2![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif) ). The imine + isocyanate products are formed upon oxygen transfer from nitrones to isocyanides, and N–O bond cleavage is the main factor determining this process. A metallacycle is formed upon decomposition of the cycloadduct, and this process includes deprotonation of the oxadiazoline CH group/N–O bond cleavage and Cl− elimination/cyclization. The main factor controlling the metallacycle formation is the acidity of the endocyclic CH group in the cycloadduct. Effects of the substituent R in C
). The imine + isocyanate products are formed upon oxygen transfer from nitrones to isocyanides, and N–O bond cleavage is the main factor determining this process. A metallacycle is formed upon decomposition of the cycloadduct, and this process includes deprotonation of the oxadiazoline CH group/N–O bond cleavage and Cl− elimination/cyclization. The main factor controlling the metallacycle formation is the acidity of the endocyclic CH group in the cycloadduct. Effects of the substituent R in C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NR and the nitrone nature on the reactivity are analysed.
NR and the nitrone nature on the reactivity are analysed.


 Please wait while we load your content...
Please wait while we load your content...