Na2.32Co1.84(SO4)3 as a new member of the alluaudite family of high-voltage sodium battery cathodes†
Abstract
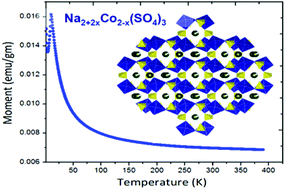
Electrochemical energy storage has recently seen tremendous emphasis being placed on the large-scale (power) grid storage. Sodium-ion batteries are capable of achieving this goal with economic viability. In a recent breakthrough in sodium-ion battery research, the alluaudite framework (Na2Fe2(SO4)3) has been reported, with the highest Fe3+/Fe2+ redox potential (ca. 3.8 V, Barpanda, et al., Nat. Commun., 2014, 5, 4358). Exploring this high-voltage sodium insertion system, we report the discovery of Na2+2xCo2−x(SO4)3 (x = 0.16) as a new member of the alluaudite class of cathode. Stabilized by low-temperature solid-state synthesis (T ≤ 350 °C), this novel Co-based compound assumes a monoclinic structure with C2/c symmetry, which undergoes antiferromagnetic ordering below 10.2 K. Isotypical to the Fe-homologue, it forms a complete family of solid-solution Na2+2x(Fe1−yCoy)2−x(SO4)3 [y = 0–1]. Ab initio DFT analysis hints at potential high voltage operation at 4.76–5.76 V (vs. Na), depending on the degree of desodiation involving a strong participation of the oxygen sub-lattice. With the development of safe organic electrolytes, Na2+2xCo2−x(SO4)3 can work as a cathode material (∼5 V) for sodium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...