Synthesis, structure and properties of new bimetallic sodium and potassium lanthanum borohydrides†
Abstract
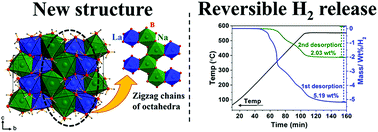
Two new bimetallic sodium or potassium lanthanum borohydrides, NaLa(BH4)4 and K3La(BH4)6, are formed using La(BH4)3 free of metal halide by-products. NaLa(BH4)4 crystallizes in an orthorhombic crystal system with unit cell parameters, a = 6.7987(19), b = 17.311(5), c = 7.2653(19) Å and space group symmetry Pbcn. This compound has a new structure type built from brucite-like layers of octahedra (hcp packing of anions) with half of the octahedral sites empty leading to octahedral chains similar to rutile (straight chains) or α-PbO2 (zig-zag chains). K3La(BH4)6 crystallizes in the monoclinic crystal system with unit cell parameters a = 7.938(2), b = 8.352(2), c = 11.571(3) Å, β = 90.19(6)° and space group P21/n with a double-perovskite type structure. Thermogravimetric analysis shows a mass loss of 5.86 and 2.83 wt% for NaLa(BH4)4 and K3La(BH4)6, respectively, in the temperature range of room temperature to 400 °C. Mass spectrometry shows that hydrogen release starts at 212 and 275 °C for NaLa(BH4)4 and K3La(BH4)6, respectively and confirms that no diborane is released. Sieverts’ measurements reveal that 2.03 and 0.49 wt% of hydrogen can be released from the NaLa(BH4)4 and K3La(BH4)6, respectively, during the second hydrogen desorption cycle at the selected physical condition for hydrogen absorption.


 Please wait while we load your content...
Please wait while we load your content...