Potassium uranyl borate 3D framework compound resulted from temperature directed hydroborate condensation: structure, spectroscopy, and dissolution studies†
Abstract
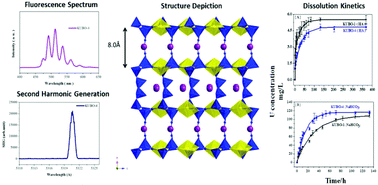
The equatorial coordination nature of the uranyl unit has resulted in only three uranyl borate 3D framework compounds reported so far formed from boric acid flux reactions conducted at 190 °C while all others are 2D layers. Here in this work, by increasing the reaction temperature to 250 °C, a new potassium uranyl borate K[(UO2)B6O10(OH)] (KUBO-4) framework compound is synthesized that shares the same layer topology with the previously reported 2D layered KUBO-1. The 3D structure of KUBO-4 is achieved by interlayer hydroborate condensation. The KUBO-4 was further characterized with single crystal XRD, SHG and fluorescence spectra, and TG/DSC measurements. A deep understanding regarding the dissolution behaviours of uranyl borate is achieved via solubility studies of the KUBO-1 and KUBO-4 performed using a combination of ICP-MS, powder XRD, and fluorescence spectroscopy techniques. The results confirm the lack of stability of borates in aqueous solutions with the presence of coordinating ligands in the environment regardless of the structure types.

 Please wait while we load your content...
Please wait while we load your content...