One dimensional metal dithiolene (M = Ni, Fe, Zn) coordination polymers for the hydrogen evolution reaction†
Abstract
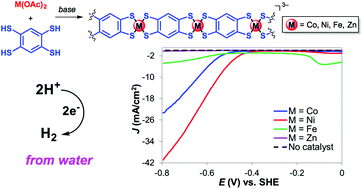
Immobilization of metal complexes to electrode surfaces has emerged as an attractive strategy to combine homogeneous and heterogeneous catalysis. We recently reported the immobilization of cobalt dithiolene catalytic units via incorporation into extended one and two dimensional (1D and 2D) frameworks. We extend here this methodology to the formation of 1D nickel, iron, and zinc dithiolene coordination polymers based on benzene-1,2,4,5-tetrathiolate (BTT) frameworks and investigate their catalytic H2-evolving activities under fully aqueous conditions. The nickel dithiolene coordination polymer is an active electrocatalyst for the hydrogen evolution reaction (HER). An overpotential of 470 mV was required to reach a current density of 10 mA cm−2 at pH 1.3, making this system one of the best performing heterogenized molecular catalysts for HER. This overpotential is 90 mV lower than that of the cobalt analogue, suggesting that the nickel coordination polymer is a more efficient H2-evolving catalyst. Additionally, no decrease in activity is observed for the nickel polymer during the first hour of electrolysis, indicating that it is stable under prolonged electrolysis.

 Please wait while we load your content...
Please wait while we load your content...