Mono-nuclear copper complexes mimicking the intermediates for the binuclear copper center of the subunit II of cytochrome oxidase: a peptide based approach†
Abstract
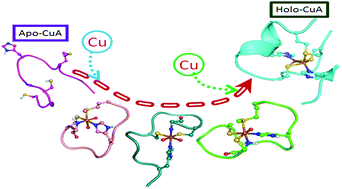
Three stable copper complexes of peptides derived from the copper ion binding loop of the subunit II of cytochrome c oxidase have been prepared and characterized by various spectroscopic techniques. These stable copper complexes of peptides were found to exhibit cysteine, histidine and/or methionine ligation, which has predominant σ-contribution in the Cys–Cu charge transfer. The copper(II) peptide complexes showed type-2 EPR spectra, which is uncommon in copper–cysteinate complexes. UV-visible spectra, Raman and EPR results support a tetragonal structure of the coordination geometry around the copper ion. The copper complex of the 9-amino acid peptide suggested the formation of a ‘red’ copper center while the copper complexes of the 12- and 11-amino acid peptides showed the formation of a ‘green’ copper center. The results provide insights on the first stable models of the copper complexes formed in the peptide scaffold that mimic the mono-nuclear copper bound protein intermediates proposed during the formation of the binuclear Cu2S2 core of the enzyme. These three copper complexes of peptides derived from the metal ion binding loop of the CuA center of the subunit II of cytochrome c oxidase showed novel spectroscopic properties which have not so far been reported in any stable small complex.


 Please wait while we load your content...
Please wait while we load your content...