Steric versus electronic factors in metallacarborane isomerisation: nickelacarboranes with 3,1,2-, 4,1,2- and 2,1,8-NiC2B9 architectures and pendant carborane groups, derived from 1,1′-bis(o-carborane)†
Abstract
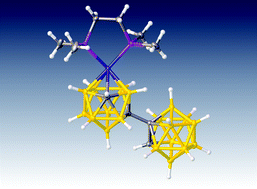
Metalation of the [7-(1′-1′,2′-closo-C2B10H11)-7,8-nido-C2B9H10]2− dianion with various {NiPP2+} or {NiP22+} fragments (PP = chelating diphosphine; P = monodentate phosphine or phosphite) leads either to unisomerised 3,1,2-NiC2B9 species or to isomerised 4,1,2-NiC2B9 or 2,1,8-NiC2B9 species, all with a pendant C2B10 substituent. The products [1-(1′-1′,2′-closo-C2B10H11)-3-dppe-3,1,2-closo-NiC2B9H10] (1), [2-(1′-1′,2′-closo-C2B10H11)-4-dppe-4,1,2-closo-NiC2B9H10] (2), [8-(1′-1′,2′-closo-C2B10H11)-2-dmpe-2,1,8-closo-NiC2B9H10] (3), [1-(1′-1′,2′-closo-C2B10H11)-3,3-(PMe3)2-3,1,2-closo-NiC2B9H10] (4), [1-(1′-1′,2′-closo-C2B10H11)-3,3-(PMe2Ph)2-3,1,2-closo-NiC2B9H10] (6), [1-(1′-1′,2′-closo-C2B10H11)-3,3-{P(OMe)3}2-3,1,2-closo-NiC2B9H10] (9) and [1-(1′-1′,2′-closo-C2B10H11)-2,2-{P(OMe)3}2-2,1,8-closo-NiC2B9H10] (10) were fully characterised spectroscopically and crystallographically, whilst [2-(1′-1′,2′-closo-C2B10H11)-4,4-(PMePh2)2-4,1,2-closo-NiC2B9H10] (8) was characterised spectroscopically. Overall the results suggest that an important factor in a 3,1,2 to 4,1,2 isomerisation is the relief gained from steric crowding, whereas a 3,1,2 to 2,1,8 isomerisation appears to be favoured by strongly electron-donating ligands on the metal.

 Please wait while we load your content...
Please wait while we load your content...