Mechanistic insights into the role of PC- and PCP-type palladium catalysts in asymmetric hydrophosphination of activated alkenes incorporating potential coordinating heteroatoms†
Abstract
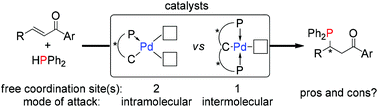
The impact of the structural attributes of chiral PC- and PCP-palladium catalysts was investigated in the asymmetric hydrophosphination of various heterocycle-functionalized enone substrates. Due to the architecture of the catalysts, they are confronted with potential catalyst deactivation arising from the coordination of the electron-rich heteroatoms (P, O, N and S) to the metal center. A systematic variation of the location and identity of the heteroatoms demonstrated the impact of structural modifications on the substrates, which have a significant influence on both yields (16–99%) and enantioselectivities (0–99%). A detailed discussion on the distinct catalytic mechanisms (intra- vs. inter-molecular addition) provides important information to explain the results obtained.

 Please wait while we load your content...
Please wait while we load your content...