A comparison of MOP-phosphonite ligands and their applications in Rh(i)- and Pd(ii)-catalysed asymmetric transformations†
Abstract
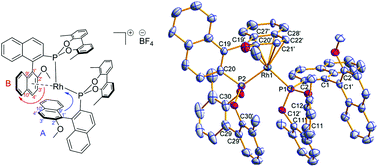
Six chiral MOP-phosphonites have been synthesised and compared via experimental and computational methods in an effort to quantify their differing structural and electronic profiles. They were found to be electron-poor ligands in comparison to their arylphosphine analogues and have a larger trans influence in square planar Pt(II) complexes. Four [Rh(LP)(η2:η2-cod)Cl] complexes were synthesised and characterised by NMR, HRMS and X-ray crystallography. Two [Rh(LP)2]BF4 complexes were prepared where one ligand acts as a chelating P,C-π-donor; detailed NMR studies demonstrated a hemilabile η6-coordination mode, which in one case was confirmed by X-ray crystallography. Rh(I) complexes were used as catalysts in asymmetric hydrogenation and hydroformylation reactions and in the addition of phenyl boronic acid to an isatin. Pd(II) complexes were successfully employed in asymmetric Suzuki–Miyaura cross-coupling reactions yielding binaphthyl products. Two [Pd(LP)2Cl2] complexes were synthesised and characterised by X-ray crystallography, both adopting cis orientations, with one of the complexes crystallising as two pseudo-polymorphs.

 Please wait while we load your content...
Please wait while we load your content...