Mono/bimetallic water-stable lanthanide coordination polymers as luminescent probes for detecting cations, anions and organic solvent molecules†
Abstract
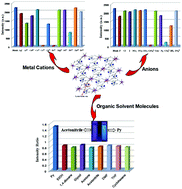
Eleven water-stable isostructural mono/bimetallic lanthanide coordination polymers (Ln-CPs) {[EuxTb1−x (HL)(H2O)3]·H2O}n (x = 1.0 (1), 0.9 (3), 0.8 (4), 0.7 (5), 0.6 (6), 0.4 (7), 0.3 (8), 0.2 (9), 0.1 (10), 0.05 (11), 0 (2), H4L = 5,5′-(1H-2,3,5-triazole-1,4-diyl)diisophthalic acid) with uncoordinated Lewis basic triazole sites within the pores were prepared. The Ln-CPs represented by 1 showed a rapid and drastic emission quenching induced by external Fe3+ and Cr3+ cations and CrO42− and CO32− anions in aqueous solution. In addition, because of the comparable emission intensities of Eu3+ and Tb3+ ions, bimetallic CP 8 can be used as a ratiometric luminescent sensor for organic solvent molecules. Moreover, the luminescent color of the 8 sensor in pyridine and in other guest solvents undergoes obvious changes that can be clearly distinguished by the naked eye.

 Please wait while we load your content...
Please wait while we load your content...