Interactions between heme and tau-derived R1 peptides: binding and oxidative reactivity†
Abstract
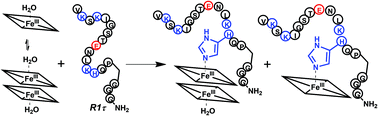
The interaction of hemin with the first 18-amino acid repeat in tau protein has been investigated at both the N-terminal free-amine (R1τ) and N-acetylated (AcR1τ) forms for its potential relevance in traumatic brain injury and possibly other neurodegenerative diseases. The binding properties of hemin-R1τ and hemin-AcR1τ were compared with those of the hemin complex with amyloid-β peptide fragment 1–16 (Aβ16) and synthetic hemins. AcR1τ and R1τ bind with moderate affinity to both monomeric and dimeric hemin to form 1 : 1 complexes, but for the acetylated peptide, the affinity is one order of magnitude larger (K1 = 3.3 × 106 M−1). The binding constants were similar to that of Aβ16 for hemin, but unlike the latter, neither of the two R1τ peptides forms a 2 : 1 complex with hemin. This is mostly due to electrostatic repulsion between R1τ chains, and in particular the C-terminal proline-15 kink, while structural features of the hemin-R1τ complexes do not seem to play a role. In fact, the same features are observed for the interaction between ferric heme and peptide R1τ*, where the P15 residue is replaced by an alanine. Imidazole neither binds to [hemin(R1τ)] nor [hemin(AcR1τ)], whereas small ligands such as CN and CO easily bind to the ferric and ferrous forms of the complexes, respectively. A detailed comparative study of the peroxidase activity of [hemin(R1τ)] and [hemin(AcR1τ)] shows that such activity is very low. Thus, the association between heme and unfolded neuronal peptides does not, per se, involve a significant gain of toxic pseudo-enzymatic activity. However, under conditions of heavy heme release occurring on traumatic brain injury or when this activity is prolonged for long time, it can contribute to neuronal oxidative stress. In addition, the presence of hemin increases the aggregation propensity of R1τ.

 Please wait while we load your content...
Please wait while we load your content...