Complexes of the tripodal phosphine ligands PhSi(XPPh2)3 (X = CH2, O): synthesis, structure and catalytic activity in the hydroboration of CO2†
Abstract
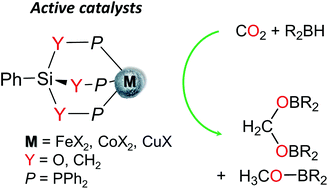
The coordination chemistry of Fe2+, Co2+ and Cu+ ions was explored with the triphosphine and triphosphinite ligands PhSi{CH2PPh2}3 (1) and PhSi{OPPh2}3 (2), so as to evaluate the impact of the electronic properties of the tripodal phosphorus ligands on the structure and reactivity of the corresponding complexes. The synthesis and characterization of the complexes [Fe(κ3-PhSi{CH2PPh2}3)(MeCN)3][OTf]2 (3) (OTf = O3SCF3), [Fe(κ3-PhSi{OPPh2}3)(MeCN)3][OTf]2 (3′), [Co(κ2-PhSi{CH2PPh2}3)Cl2] (4), [Co(κ3-PhSi{OPPh2}3)Cl2] (4′), [Cu(κ3-PhSi{CH2PPh2}3)Br] (5) and [Cu(κ3-PhSi{OPPh2}3)I] (5′) were carried out. The crystal structures of 3, 3′, 4, 4′, and of the solvates 5·3THF and 5′·THF are reported. Complexes 3–5′ were shown to promote the catalytic hydroboration of CO2 with (9-BBN)2 (9-BBN = 9-borabicyclo[3.3.1]nonane). While the iron and cobalt complexes of the triphosphine 1 are more active than the analogous complexes with 2, the opposite trend is observed with the copper catalysts. Overall, the copper catalysts 5 and 5′ are both more reactive and more selective than the Fe and Co catalysts, enabling the formation of the acetal H2C(OBBN)2 with a high molar ratio of H2C(OBBN)2 : CH3OBBN up to 92 : 8.
- This article is part of the themed collection: Small Molecule Activation

 Please wait while we load your content...
Please wait while we load your content...