Hydrolysis of thorium(iv) at variable temperatures†
Abstract
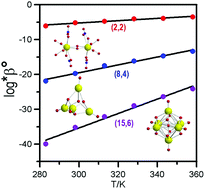
Hydrolysis of Th(IV) was studied in tetraethylammonium perchlorate (0.10 mol kg−1) at variable temperatures (283–358 K) by potentiometry and microcalorimetry. Three hydrolysis reactions, mTh4+ + nH2O = Thm(OH)n(4m−n)+ + nH+, in which (n,m) = (2,2), (8,4), and (15,6), were invoked to describe the potentiometric and calorimetric data for solutions with the [hydroxide]/[Th(IV)] ratio ≤ 2. At higher ratios, the formation of (16,5) cannot be excluded. The hydrolysis constants, *β2,2, *β8,4, and *β15,6, increased by 3, 7, and 11 orders of magnitude, respectively, as the temperature was increased from 283 to 358 K. The enhancement is mainly due to the significant increase of the degree of ionization of water as the temperature rises. All three hydrolysis reactions are endothermic at 298 K, with enthalpies of (118 ± 4) kJ mol−1, (236 ± 7) kJ mol−1, and (554 ± 4) kJ mol−1 for ΔH2,2, ΔH8,4, and ΔH15,6 respectively. The hydrolysis constants at infinite dilution have been obtained with the specific ion interaction approach. The applicability of three approaches for estimating the equilibrium constants at different temperatures, including the constant enthalpy approach, the constant heat capacity approach and the DQUANT equation was evaluated with the data from this work.

 Please wait while we load your content...
Please wait while we load your content...