Luminescence behaviour of Mn4+ ions in seven coordination environments of K3ZrF7
Abstract
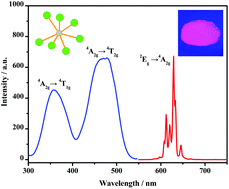
A single-phased Mn4+ doped fluorozirconate red phosphor, K3ZrF7:Mn4+, has been successfully synthesized. Its structure, morphology, composition and optical properties were investigated by X-ray diffraction, scanning electron microscopy, energy-dispersive X-ray spectroscopy, atomic absorption spectroscopy, diffuse reflectance spectroscopy, photoluminescence spectroscopy and by using luminescence decay curves. It was found that Mn4+ ions only coordinating with seven F− anions in a K3ZrF7 crystal field can possess intense red emission under blue light illumination. Mixing the obtained K3ZrF7:Mn4+ red phosphor with commercial Y3Al5O12:Ce3+ and coating the mixture on a blue-GaN chip, obvious warm white light with a low correlated color temperature (2970 K) and a high color rendering index (Ra = 91.4 and R9 = 72) were achieved from white light-emitting diode devices.

 Please wait while we load your content...
Please wait while we load your content...