Synthesis, characterization and biological evaluation of labile intercalative ruthenium(ii) complexes for anticancer drug screening†
Abstract
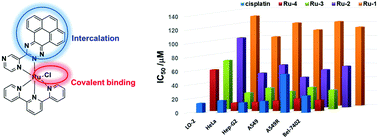
DNA binding and DNA transcription inhibition is regarded as a promising strategy for cancer chemotherapy. Herein, chloro terpyridyl Ru(II) complexes, [Ru(tpy)(N^N)Cl]+ (Ru1, N^N = 2,2′-bipyridine; Ru2, N^N = 3-(pyrazin-2-yl)-as-triazino[5,6-f]acenaphthylene; Ru3, N^N = 3-(pyrazin-2-yl)-as-triazino[5,6-f]phenanthrene; Ru4, N^N = 3-(pyrazin-2-yl)-as-triazino[5,6-f]pyrene) were prepared as DNA intercalative and covalent binding anticancer agents. The chloro ligand hydrolysis slowly and the octanol and water partition coefficient of Ru2–Ru4 were between 0.6 and 1.2. MALDI-TOF mass, DNA gel electrophoresis confirmed covalent and intercalative DNA binding modes of Ru2–Ru4, while Ru1 can only bind DNA covalently. As a result, Ru2–Ru4 exhibited stronger DNA transcription inhibition activity, higher cell uptake efficiency and better anticancer activity than Ru1. Ru4 was the most toxic complex toward all cancer cells which inhibited DNA replication and transcription. AO/EB, Annexin V/PI, nuclear staining, JC-1 assays further confirmed that Ru2–Ru4 induced cancer cell death by an apoptosis mechanism.
- This article is part of the themed collection: Metallodrugs: Activation, Targeting, and Delivery

 Please wait while we load your content...
Please wait while we load your content...