Polypyrrole/cobalt ferrite/multiwalled carbon nanotubes as an adsorbent for removing uranium ions from aqueous solutions†
Abstract
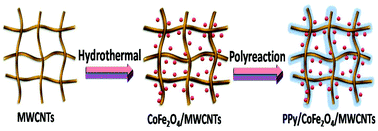
A novel rod-like, dual-shell structural adsorbent of polypyrrole/cobalt ferrite/multiwalled carbon nanotubes (PPy/CoFe2O4/MWCNTs) was successfully synthesized by a hydrothermal method, which could easily separate uranium(VI) ions with an external magnetic field. The structure and morphology of PPy/CoFe2O4/MWCNTs were characterized by VSM, XRD, XPS TEM and FT-IR. The results proved that the dual-shell structure was obtained in which a shell of cobalt ferrite and polypyrrole formed around the MWCNTs core. In batch adsorption experiments, including pH, equilibrium time and temperature on uranium adsorption, were investigated. The main results show that the PPy/CoFe2O4/MWCNTs composite has a higher affinity towards the uptake of uranium(VI) from aqueous solutions. The highest adsorption capacity reached was 148.8 mg U per g at pH 7. A kinetic analysis showed that the adsorption process was best described by a pseudo-second-order kinetic model. The uranium sorption equilibrium data correlated well with the Langmuir sorption isotherm model in the thermodynamic analysis. 0.5 mol per L NaHCO3 was used as the desorbent and good adsorption properties were shown after the desorption procedures were repeated three times. Thus, PPy/CoFe2O4/MWCNTs was an excellent adsorbent for removing uranium(VI) ions.

 Please wait while we load your content...
Please wait while we load your content...