N2S2 and N4S4 precursors to PS2 macrocycles and cyclic amidinium salts†
Abstract
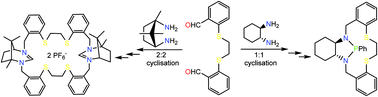
The cyclo-condensation of 1R,2R-diaminocyclohexane with 2,2′-(ethane-1,2-diyldisulfanediyl)dibenzaldehyde gave the 1 : 1 addition compound chxn-imN2S2 in high yield. When the same condensation reaction was performed with 1R,3S-diamino-1,7,7-trimethylcyclopentane as the diamine, the 2 : 2 addition compound tmcp-imN4S4 was obtained selectively. Reduction of the diimines gave the saturated analogues chxn-N2S2 (1) and tmcp-N4S4 (3) the former of which could be phosphorylated with PhP(NMe2)2 to give the novel 13-membered macrocycle chxn-PS2, 2. Introduction of the phenylphosphine function proved stereoselective with a preference for the N(R)/N(S) configuration at the nitrogen atoms. The coordination chemistry of the novel phosphine has been explored with Cu(I) and Mo(0) through formation of the complexes Cu(2)I, 4, and Mo(CO)3(2), 5. Extension of the phosphorylation chemistry to tmcp-N4S4 (3) proved unsuccessful but ring closure reactions of both 1 and 3 with triethylorthoformate gave cyclic amidinium salts which are potential precursors to macrocyclic N-heterocyclic carbenes.

 Please wait while we load your content...
Please wait while we load your content...