Chemical state determination of molecular gallium compounds using XPS†
Abstract
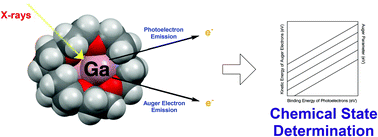
A series of molecular gallium compounds were analyzed using X-ray photoelectron spectroscopy (XPS). Specifically, the Ga 2p3/2 and Ga 3d5/2 photoelectron binding energies and the Ga L3M45M45 Auger electron kinetic energies of compounds with gallium in a range of assigned oxidation numbers and with different stabilizing ligands were measured. Auger parameters were calculated and used to generate multiple chemical speciation (or Wagner) plots that were subsequently used to characterize the novel gallium–cryptand[2.2.2] complexes 1–3 that possess ambiguous oxidation numbers for gallium. The results presented demonstrate the ability of widely accessible XPS instruments to experimentally determine the chemical state of gallium centers and, as a consequence, provide deeper insights into reactivity compared to assigned oxidation and valence numbers.

 Please wait while we load your content...
Please wait while we load your content...