New sterically-hindered o-quinones annelated with metal-dithiolates: regiospecificity in oxidative addition reactions of a bifacial ligand to the Pd and Pt complexes†
Abstract
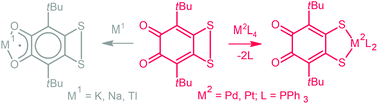
An unusual reactivity of sterically hindered o-quinones with an annelated dithiete ring towards coordination at a dithiolene site has been discovered. New Pd and Pt dithiolate complexes have been synthesized. The reaction proceeds regioselectively, and the quinone site of the parent ligand is not affected even while using an excess of the metal complex. Both Pt and Pd complexes display a square planar surrounding for the metal ion and have very similar NMR, IR and UV/Vis spectra. Surprisingly, being coordinated at the dithiolene site to the metal, the ligand exhibits activity like an o-quinone, it could be reduced with different metals resulting in the corresponding o-semiquinonates which were confirmed by EPR spectroscopy. It was shown that an unpaired electron exhibits HFC with the phosphorus nuclei of phosphine ligands coordinated to the metal ions at the dithiolene site of the molecule.


 Please wait while we load your content...
Please wait while we load your content...