Neutral copper(i) complexes featuring phosphinesulfonate chelates†
Abstract
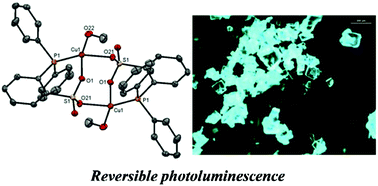
The reaction of diphenylphosphinobenzenesulfonic acid with copper(I) oxide resulted in the formation of the new neutral dimeric copper(I) complex {Cu2(DPPBS)2·(MeOH)2}. X-ray diffraction studies revealed that the complex has a dimeric structure and a pyramidal trigonal geometry around the copper atom which contains coordinated methanol molecules at the copper centers. Cleavage of the dimer by reaction with various bipyrimidines enabled the preparation of the corresponding well-defined heterotopic mononuclear [Cu(P^O)(N^N)] and dinuclear {(P^O)Cu(N^N)Cu(P^O)} complexes. X-ray crystal structure determination shows these to have distorted tetrahedral geometries. Their absorption and emission properties were investigated experimentally and photophysical data were also confirmed by DFT and TD-DFT calculations. Owing to the methanol molecules, the neutral crystalline dimer {Cu2(DPPBS)2·(MeOH)2} displays green reversible photoluminescence upon UV irradiation in the solid state with an absolute luminescence quantum yield of 0.51.

 Please wait while we load your content...
Please wait while we load your content...