Thermoelectric properties of double-substituted tetrahedrites Cu12−xCoxSb4−yTeyS13
Abstract
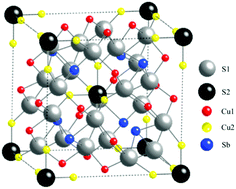
Tetrahedrites, a class of earth-abundant minerals, exhibit extremely low thermal conductivity values making them promising candidates for thermoelectric applications at high temperatures. Herein, we extend investigations on these materials to specimens substituted on both the Cu and Sb sites by reporting on the thermoelectric properties of polycrystalline Cu12−xCoxSb4−yTeyS13 in a wide range of temperatures (2–700 K). All prepared samples exhibit p-type heavily-doped semiconducting behavior with relatively low electrical resistivity values. These substitutions have little influence on the thermal conductivity, which remains very low in the whole temperature range (∼0.7 W m−1 K−1). Although the double-substituted samples exhibit higher ZT values with respect to the parent tetrahedrite Cu12Sb4S13, the maximum ZT of 0.80 reached at 700 K for (x,y) = (0.82, 0.41) remains comparable to the values obtained in compounds solely substituted with Co or Te.

 Please wait while we load your content...
Please wait while we load your content...