Aryl(silyl)amino group stabilized hydridosilanediols: synthesis and characterization and use for preparation of alumino(hydrido)siloxanes†
Abstract
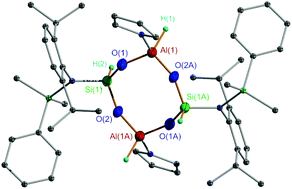
Aryl(silyl)amino group stabilized hydridosilanediols RSiH(OH)2 (R = N(SiMe2Ph)-2,6-iPr2C6H3 (4), N(SiMe3)-2,6-iPr2C6H3 (5), and N(SiMe2Ph)-2,4,6-Me3C6H2 (6)) were prepared from the controlled hydrolysis of the related RSiHCl2 (1–3) each in the presence of aniline as the HCl acceptor. Reactions of 4 with AlMe3, AliBu3, AlH(iBu)2, and AlH3·NMe3, respectively, yielded alumino(hydrido)siloxanes [2,6-iPr2C6H3N(SiMe2Ph)Si(H)OAlMe(THF)]2 (7), [2,6-iPr2C6H3N(SiMe2Ph)Si(H)OAliBu(THF)]2 (9), [2,6-iPr2C6H3N(SiMe2Ph)Si(H)O2]3[Al(THF)]2 (10), and [2,6-iPr2C6H3N(SiMe2Ph)Si(H)OAlH(THF)]2 (11). The reaction of 5 with AlMe3 gave [2,6-iPr2C6H3N(SiMe3)Si(H)OAlMe(THF)]2 (8), a compound similar to 7. Compounds 1–11 are characterized by NMR (1H, 13C, and 29Si) and IR spectroscopy and CHN elemental analysis, of which 6 and 8–11 are further studied by X-ray crystallography. Compounds 8–9 and 11 feature cyclic structures all with the skeleton core of Si2O4Al2 while compound 10 exhibits a bicyclic structure having a core of Si3O6Al2. Melting point measurements indicated that 4–6 are thermally stable bearing the geminal SiH and SiOH groups. Compounds 8–9 and 11 are thermally stable as well with the O atom-bridged SiH and AlR (R = Me, iBu, or H) groups.

 Please wait while we load your content...
Please wait while we load your content...