Interaction of europium and curium with alpha-amylase†
Abstract
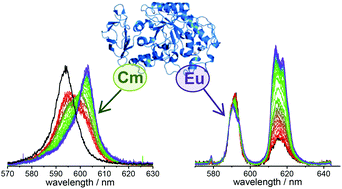
The complexation of Eu(III) and Cm(III) with the protein α-amylase (Amy), a major enzyme in saliva and pancreatic juice, was investigated over wide ranges of pH and concentration at both ambient and physiological temperatures. Macroscopic sorption experiments demonstrated a strong and fast binding of Eu(III) to Amy between pH 5 and 8. The protein provides three independent, non-cooperative binding sites for Eu(III). The overall association constant of these three binding sites on the protein was calculated to be log K = 6.4 ± 0.1 at ambient temperature. With potentiometric titration, the averaged deprotonation constant of the carboxyl groups (the aspartic and glutamic acid residues) of Amy was determined to be pKa = 5.23 ± 0.14 at 25 °C and 5.11 ± 0.24 at 37 °C. Time-resolved laser-induced fluorescence spectroscopy (TRLFS) revealed two different species for both Eu(III) and Cm(III) with Amy. In the case of the Eu(III) species, the stability constants were determined to be log β11 = 4.7 ± 0.2 and log β13 = 12.0 ± 0.4 for Eu : Amy = 1 : 1 and 1 : 3 complexes, respectively, whereas the values for the respective Cm(III) species were log β11 = 4.8 ± 0.1 and log β13 = 12.1 ± 0.1. Furthermore, the obtained stability constants were extrapolated to infinite dilution to make our data compatible with the existing thermodynamic database.

 Please wait while we load your content...
Please wait while we load your content...