Np(v) complexation with carbonate in aqueous solutions studied by spectrophotometric titration at various temperatures†
Abstract
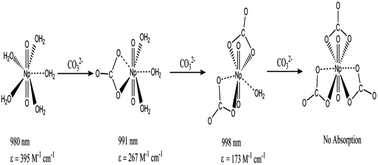
The complexation of neptunium(V) with carbonate has been studied at temperatures from 10 to 70 °C in 0.1 M LiClO4 by spectrophotometry. Three NpO2+–CO32− complex species, NpO2(CO3)n(2n−1)− (n = 1, 2, 3), are identified and the stability constants are calculated by using the absorption spectra in the near-IR region collected from titrations at varying temperatures. The enthalpies and entropies are calculated with van't Hoff equations in the temperature range of 10 to 70 °C, indicating that the formation of all NpO2+–CO32− complexes is mainly entropy driven. The structures of the NpO2+–CO32− complex species in aqueous solutions are also reviewed. Based on the molar absorptivity of Np(V) in the near-IR region the structure of NpO2(CO3)23− is re-constructed as NpO2(CO3)2(H2O)3−of low symmetry but not as NpO2(CO3)2(H2O)23−of high symmetry as suggested in a previous study.

 Please wait while we load your content...
Please wait while we load your content...