The role of imidoselenium(ii) chlorides in the formation of cyclic selenium imides via cyclocondensation†
Abstract
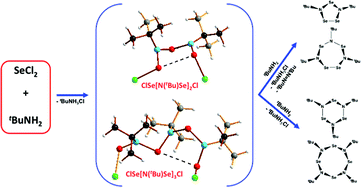
The third member of the series of imidoselenium(II) chlorides ClSe[N(tBu)Se]nCl (n = 3) (9) has been isolated from the cyclocondensation reaction of tBuNH2 and SeCl2 in THF in a molar ratio of ca. 3 : 1 and characterized in the form of two polymorphs 9a and 9b by single crystal X-ray analysis. The unusual structural features of this nine-atom chain are explained satisfactorily in terms of a bonding model that invokes intra-molecular secondary bonding interactions and hyperconjugation. The reaction of the bifunctional reagent ClSe[N(tBu)Se]2Cl (8) with tBuNH2 in THF occurs via concurrent pathways to give 1,3,5-Se3(NtBu)3 (1) and 1,3-Se3(NtBu)2 (3a). The energetics of the reactions of tBuNH2 and SeCl2 in THF have been calculated at the PBE0/def2-TZVPP level of theory in order to assess the feasibility of ClSe[N(tBu)Se]nCl (7–9, n = 1–3) as intermediates in the formation of known cyclic selenium imides. DFT calculations were also employed to explore the energy profile of the pathway of the formation of the first member of the series ClSeN(tBu)SeCl (7) from tBuNH2 and SeCl2 in THF at 298 K. The neutral ligand ClSeN(tBu)SeCl (7) is Se,Se′-coordinated to the metal centre in the unusual adduct [PdCl2{Se,Se′-(SeCl)2N(tBu)}]·[PdCl2{Se,Se′-Se4(NtBu)3}]·MeCN (10·MeCN), which is the first metal complex of an imidoselenium(II) chloride.
- This article is part of the themed collections: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST and Main Group Transformations

 Please wait while we load your content...
Please wait while we load your content...