Accessible heavier s-block dihydropyridines: structural elucidation and reactivity of isolable molecular hydride sources†
Abstract
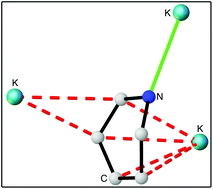
The straightforward metathesis of 1-lithio-2-tbutyl-1,2-dihydropyridine using metal tert-butoxide (Na/K) has resulted in the first preparation and isolation of a series of heavier alkali metal dihydropyridines. By employing donors, TMEDA, PMDETA and THF, five new metallodihydropyridine compounds were isolated and fully characterised. Three distinct structural motifs have been observed; a dimer, a dimer of dimers and a novel polymeric dihydropyridylpotassium compound, and the influence of cation π-interactions therein has been discussed. Thermal volatility analysis has shown that these complexes have the potential to be used as simple isolable sodium or potassium hydride surrogates, which is confirmed in test reactions with benzophenone.
- This article is part of the themed collection: Main Group Transformations

 Please wait while we load your content...
Please wait while we load your content...