Preparation of α1- and α2-isomers of mono-Ru-substituted Dawson-type phosphotungstates with an aqua ligand and comparison of their redox potentials, catalytic activities, and thermal stabilities with Keggin-type derivatives†
Abstract
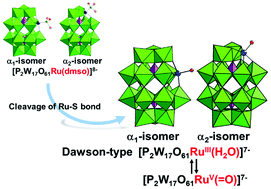
Both the α1- and the α2-isomers of mono-ruthenium (Ru)-substituted Dawson-type phosphotungstates with terminal aqua ligands, [α1-P2W17O61RuIII(H2O)]7− (α1-RuH2O) and [α2-P2W17O61RuIII(H2O)]7− (α2-RuH2O), were prepared in pure form by cleavage of the Ru–S bond of the corresponding DMSO derivatives, [α1-P2W17O61Ru(DMSO)]8− (α1-RuDMSO) and [α2-P2W17O61Ru(DMSO)]8− (α2-RuDMSO), respectively. Redox studies indicated that α1-RuH2O and α2-RuH2O show proton-coupled electron transfer (PCET), and the RuIII(H2O) species was reversibly reduced to RuII(H2O) species and oxidized to RuIV(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) species and further to RuV(
O) species and further to RuV(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) species in aqueous solution depending on the pH. Their redox potentials and thermal stabilities were compared with those of the corresponding α-Keggin-type derivatives ([α-XW11O39Ru(H2O)]n−; X = Si4+ (n = 5), Ge4+ (n = 5), or P5+ (n = 4)). The basic electronic and redox features of Ru(L)-substituted Keggin- and Dawson-type heteropolytungstates (with L = H2O or O2−) were analyzed by means of density functional calculations. Similar to the corresponding α-Keggin-type derivatives, both α1-RuH2O and α2-RuH2O show catalytic activity for water oxidation.
O) species in aqueous solution depending on the pH. Their redox potentials and thermal stabilities were compared with those of the corresponding α-Keggin-type derivatives ([α-XW11O39Ru(H2O)]n−; X = Si4+ (n = 5), Ge4+ (n = 5), or P5+ (n = 4)). The basic electronic and redox features of Ru(L)-substituted Keggin- and Dawson-type heteropolytungstates (with L = H2O or O2−) were analyzed by means of density functional calculations. Similar to the corresponding α-Keggin-type derivatives, both α1-RuH2O and α2-RuH2O show catalytic activity for water oxidation.

 Please wait while we load your content...
Please wait while we load your content...