First-row transition metal complexes of ENENES ligands: the ability of the thioether donor to impact the coordination chemistry†
Abstract
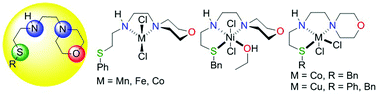
The reactions of two variants of ENENES ligands, E(CH2)2NH(CH)2SR, where E = 4-morpholinyl, R = Ph (a), Bn (b) with MCl2 (M = Mn, Fe, Co, Ni and Cu) in coordinating solvents (MeCN, EtOH) affords isolable complexes, whose magnetic susceptibility measurements suggest paramagnetism and a high-spin formulation. X-Ray diffraction studies of available crystals show that the ligand coordinates to the metal in either a bidentate κ2[N,N′] or tridentate κ3[N,N′,S] fashion, depending on the nature of ligand and/or identity of the metal atom. In the case of a less basic SPh moiety, a bidentate coordination mode was identified for harder metals (Mn, Fe), whereas a tridentate coordination mode was identified in the case of a more basic SBn moiety with softer metals (Ni, Cu). In the intermediate case of Co, ligands a and b coordinate via κ2[N,N′] and κ3[N,N′,S] coordination modes, which can be conveniently predicted by DFT calculations. For the softest metal (Cu), ligand a coordinates in a κ3[N,N′,S] fashion.

 Please wait while we load your content...
Please wait while we load your content...