Crystal structure evolution and luminescence properties of color tunable solid solution phosphors Ca2+xLa8−x(SiO4)6−x(PO4)xO2:Eu2+
Abstract
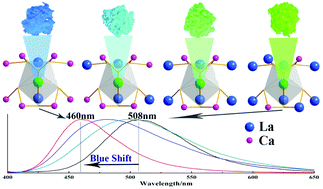
A series of apatite solid solution phosphors Ca2+xLa8−x(SiO4)6−x(PO4)xO2:Eu2+ (x = 0,2,4,6) were synthesized by a conventional high-temperature solid-state reaction. The phase purity was examined using XRD, XPS and XRF. The crystal structure information, such as the concentration, cell parameters and occupation rate, was analyzed using a Rietveld refinement, demonstrating that the Eu2+ activated the Ca2La8(SiO4)6O2 and Ca8La2(PO4)6O2 to form continuous solid solution phosphors. Different behaviors of luminescence evolution in response to structural variation were verified among the series of phosphors. Two kinds of Eu2+ ion sites were proved using low temperature PL spectra (8k) and room temperature decay curves. The substitution of large La3+ ions by small Ca2+ ions induced a decreased crystal field splitting of the Eu2+ ions, which caused an increase in emission energy from the 5d excited state to the 4f ground state and a resultant blue-shift from 508 nm to 460 nm. Therefore, with the crystal structure evolution, the emitted color of the series of phosphors could be tuned from green to blue by adjusting the ratio of Ca/La.


 Please wait while we load your content...
Please wait while we load your content...