Silver complexation by metallacryptates†
Abstract
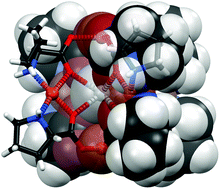
We report the first complete characterization of metallycryptates encapsulating Ag(I) cations: carboxylato ligands derived from L-proline and L-alanine chelate and bridge six Cu(II) centres arranged in a slightly distorted octahedral fashion. Eight oxygen atoms of these ligands are disposed in square-prismatic geometry and coordinate the monovalent cation. Two alternative metallacryptates based on alanine have been identified which differ with respect to aggregation: a solid in which pairs of encapsulating sites are formed competes with an infinite chain of M(I) coordinating sites. In contrast, the individual encrypting moieties are arranged as overall neutral and isolated molecular species in the proline-based metallacryptate. This proline derivative can accomodate Ag(I) and Na(I) cations and form a solid solution. Susceptibility measurements confirm ferromagnetic interactions between the Cu(II) within the hexanuclear proline cryptate and thus underline the similarity between solids accommodating Na(I) and Ag(I). Spectroscopic results suggest that these metallacryptates hardly dissociate in methanol solution.

 Please wait while we load your content...
Please wait while we load your content...