Oxidation of phenyl and hydride ligands of bis(pentamethylcyclopentadienyl)hafnium derivatives by nitrous oxide via selective oxygen atom transfer reactions: insights from quantum chemistry calculations†
Abstract
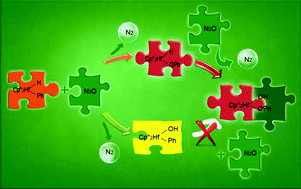
The mechanisms for the oxidation of phenyl and hydride ligands of bis(pentamethylcyclopentadienyl)hafnium derivatives 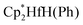 (Cp* = η5-C5Me5) by nitrous oxide via selective oxygen atom transfer reactions have been systematically studied by means of density functional theory (DFT) calculations. On the basis of the calculations, we investigated the original mechanism proposed by Hillhouse and co-workers for the activation of N2O. The calculations showed that the complex with an initial O-coordination of N2O to the coordinatively unsaturated Hf center is not a local minimum. Then we proposed a new reaction mechanism to investigate how N2O is activated and why N2O selectively oxidize phenyl and hydride ligands of
(Cp* = η5-C5Me5) by nitrous oxide via selective oxygen atom transfer reactions have been systematically studied by means of density functional theory (DFT) calculations. On the basis of the calculations, we investigated the original mechanism proposed by Hillhouse and co-workers for the activation of N2O. The calculations showed that the complex with an initial O-coordination of N2O to the coordinatively unsaturated Hf center is not a local minimum. Then we proposed a new reaction mechanism to investigate how N2O is activated and why N2O selectively oxidize phenyl and hydride ligands of 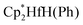 . Frontier molecular orbital theory analysis indicates that N2O is activated by nucleophilic attack by the phenyl or hydride ligand. Present calculations provide new insights into the activation of N2O involving the direct oxygen atom transfer from nitrous oxide to metal–ligand bonds instead of the generally observed oxygen abstraction reaction to generate metal–oxo species.
. Frontier molecular orbital theory analysis indicates that N2O is activated by nucleophilic attack by the phenyl or hydride ligand. Present calculations provide new insights into the activation of N2O involving the direct oxygen atom transfer from nitrous oxide to metal–ligand bonds instead of the generally observed oxygen abstraction reaction to generate metal–oxo species.

 Please wait while we load your content...
Please wait while we load your content...