Synthesis of P-stereogenic diarylphosphinic amides by directed lithiation: transformation into tertiary phosphine oxides via methanolysis, aryne chemistry and complexation behaviour toward zinc(ii)†
Abstract
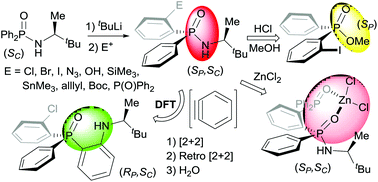
The highly diastereoselective synthesis of P-stereogenic phosphinic amides via directed ortho lithiation (DoLi) of (SC)-P,P-diphenylphosphinic amides with t-BuLi followed by electrophilic quench reactions is described. Functionalised derivatives containing a wide variety of ortho substituents (Cl, Br, I, OH, N3, SiMe3, SnMe3, P(O)Ph2, Me, allyl, tBuOCO) have been prepared in high yields with diastereomeric ratios up to 98 : 2. The X-ray diffraction structure of the ortho-stannylated and ortho-iodo compounds showed that the pro-S P-phenyl ring was stereoselectively ortho-deprotonated by the organolithium base. The usefulness of the method is supported by two key transformations, the synthesis of P-stereogenic methyl phosphinates through replacement of the chiral auxiliary by a methoxy group and the first example of the insertion of benzyne into the P–N bond of a P-stereogenic phosphinic amide. A DFT study of this reaction showed that the insertion proceeds through a [2 + 2] cycloaddition and a subsequent ring-opening with retention of the P-configuration. Explorative coordination chemistry of the new P-stereogenic ligands provided access to a chiral phosphinic amide-phosphine oxide Zn(II) complex, the crystal structure of which is reported.
- This article is part of the themed collection: Phosphorus Chemistry: Discoveries and Advances

 Please wait while we load your content...
Please wait while we load your content...