On the energetics of P–P bond dissociation of sterically strained tetraamino-diphosphanes†
Abstract
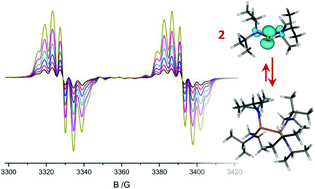
The homolytic P–P bond fission in a series of sterically congested tetraaminodiphosphanes (R2N)2P–P(NR2)2 ({4}2–{9}2, two of which were newly synthesized and fully characterized) into diaminophosphanyl radicals (R2N)2P˙ (4–9) was monitored by VT EPR spectroscopy. Determination of the radical concentration from the EPR spectra permitted to calculate free dissociation energies ΔG295Diss as well as dissociation enthalpies ΔHDiss and entropies ΔSDiss, respectively. Large positive values of ΔG295Diss indicate that the degree of dissociation is in most cases low, and the concentration of persistent radicals – even if they are spectroscopically observable at ambient temperature – remains small. Appreciable dissociation was established only for the sterically highly congested acyclic derivative {9}2. Analysis of the trends in experimental data in connection with DFT studies indicate that radical formation is favoured by large entropy contributions and the energetic effect of structural relaxation (geometrical distortions and conformational changes in acyclic derivatives) in the radicals, and disfavoured by attractive dispersion forces. Comparison of the energetics of formation for CC-saturated N-heterocyclic diphosphanes and the 7π-radical 3c indicates that the effect of energetic stabilization by π-electron delocalization in the latter is visible, but stands back behind those of steric and entropic contributions. Evaluation of spectroscopic and computational data indicates that diaminophosphanyl radicals exhibit, in contrast to aminophosphenium cations, no strong energetic preference for a planar arrangement of the (R2N)2P unit.
- This article is part of the themed collection: Phosphorus Chemistry: Discoveries and Advances

 Please wait while we load your content...
Please wait while we load your content...