Copper and nickel bind via two distinct kinetic mechanisms to a CsoR metalloregulator
Abstract
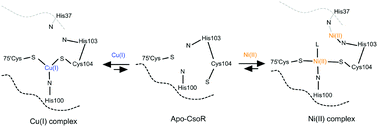
The intricate interplay between polypeptide and metal ion binding underscores many of life's fundamental processes. Metalloregulators recognise and bind cognate metal ions during cellular metal stress, evoking a transcriptional response so as to maintain metal ion homeostasis. Members of the copper sensitive operon repressor (CsoR) family of metalloregulators bind to their operator DNA in the absence of a bound metal ion, but on binding Cu(I) an allosteric conformational switch is induced that causes dissociation of the bound DNA. Other divalent metal ions are capable of binding to CsoR members but do not induce the allosteric response observed with Cu(I). The thermodynamics of Cu(I) binding has been studied in this family of metalloregulators, but the binding kinetics and mechanism of Cu(I) or a non-cognate metal ion is unknown. In the present study we have used stopped-flow absorbance kinetics and site-directed variants of the CsoR from Streptomyces lividans to monitor binding of Cu(I) and non-cognate Ni(II). The variants have been designed to individually replace known metal ion binding ligands and also to test the role of a histidine residue (His103) close, but not considered part of the Cu(I) first coordination sphere. Cu(I)/Ni(II) ion displacement studies have also been investigated. The kinetic data are most consistent with the existence of two distinct mechanisms that account for Cu(I) and Ni(II) ion binding to this CsoR. In particular Ni(II) has two binding sites; one that has identical amino acid coordination as the Cu(I) binding site and the second involving His103, a residue determined here not to be involved in the mechanism of Cu(I) binding.

 Please wait while we load your content...
Please wait while we load your content...