CoxC encased in carbon nanotubes: an efficient oxygen reduction catalyst under both acidic and alkaline conditions†
Abstract
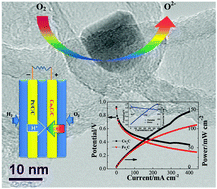
The design of a non-precious metal oxygen reduction reaction (ORR) catalyst of high activity and long durability in acidic electrolyte is of great importance for the development and commercialization of low-temperature fuel cells, which remains a great challenge to date. Here, we demonstrate a facile, scalable protocol for the controlled synthesis of CoxC encapsulated in carbon nanotubes as a novel kind of efficient electrochemical oxygen reduction reaction (ORR) catalyst. The synthesized CoxC/carbon nanotube features a high BET surface area, large pore volume and high graphitic content, which greatly favors enhanced ORR properties. The resultant composite electro-catalyst shows high ORR activity which is comparable with that of 20 wt% Pt/C in 0.1 M KOH electrolyte. More importantly, it also exhibits a high ORR activity in 0.1 M HClO4 with a near-complete 4e pathway. More attractively, compared to the most investigated FexC, CoxC as the proposed main catalytically active center shows much enhanced activity in acidic electrolyte, which will pave the way towards the rational design of an advanced electro-catalyst for an efficient ORR process especially under acidic conditions. Moreover, a fuel cell using the synthesized CoxC/carbon nanotube as a cathode catalyst showed a large open-circuit potential, high output power density and long durability, which make it a promising alternative to Pt/C as a non-precious metal ORR catalyst in proton exchange membrane fuel cells.

 Please wait while we load your content...
Please wait while we load your content...