Insights into the dehydrogenation reaction process of a K-containing Mg(NH2)2–2LiH system†
Abstract
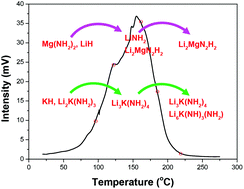
The thermal dehydrogenation process of the KOH-containing Mg(NH2)2–2LiH system was systematically investigated by identifying changes in the structure and composition of its components by XRD and FTIR. During ball milling, the added KOH reacts with Mg(NH2)2 and LiH to produce MgO, KH and Li2K(NH2)3. During the initial heating process (<120 °C), the newly formed KH and Li2K(NH2)3 react with Mg(NH2)2 and LiH to yield MgNH, LiNH2 and Li3K(NH2)4 along with hydrogen release. Raising the temperature to 185 °C results in a reaction between Mg(NH2)2, MgNH and LiH that gives Li2Mg2N3H3 as the product and further releases hydrogen. As the temperature is increased to 220 °C, Li2Mg2N3H3 reacts with LiNH2 and LiH to produce Li2MgN2H2 and H2. Meanwhile, two parallel reactions between Li2Mg2N3H3, Li3K(NH2)4 and LiH also generate additional hydrogen. Specifically, the KH and Li2K(NH2)3, formed in situ during ball milling, serve as reactants in the dehydrogenation reaction of the Mg(NH2)2–2LiH system, which is responsible for the significantly improved thermodynamics and kinetics of hydrogen storage.

 Please wait while we load your content...
Please wait while we load your content...