How a novel tyrosine–heme cross-link fine-tunes the structure and functions of heme proteins: a direct comparitive study of L29H/F43Y myoglobin†
Abstract
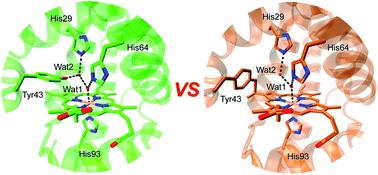
A heme–protein cross-link is a key post-translational modification (PTM) of heme proteins. Meanwhile, the structural and functional consequences of heme-protein cross-links are not fully understood, due to limited studies on a direct comparison of the same protein with and without the cross-link. A Tyr–heme cross-link with a C–O bond is a newly discovered PTM of heme proteins, and is spontaneously formed in F43Y myoglobin (Mb) between the Tyr hydroxyl group and the heme 4-vinyl group in vivo. In this study, we found that with an additional distal His29 introduced in the heme pocket, the double mutant L29H/F43Y Mb can form two distinct forms under different protein purification conditions, with and without a novel Tyr–heme cross-link. By solving the X-ray structures of both forms of L29H/F43Y Mb and performing spectroscopic studies, we made a direct structural and functional comparison in the same protein scaffold. It revealed that the Tyr–heme cross-link regulates the heme distal hydrogen-bonding network, and fine-tunes not only the spectroscopic and ligand binding properties, but also the protein reactivity. Moreover, the formation of the Tyr–heme cross-link in the double mutant L29H/F43Y Mb was investigated in vitro. This study addressed the key issue of how Tyr–heme cross-link fine-tunes the structure and functions of the heme protein, and provided a plausible mechanism for the formation of the newly discovered Tyr–heme cross-link.

 Please wait while we load your content...
Please wait while we load your content...