Theoretical insights into the separation of Am(iii) over Eu(iii) with PhenBHPPA†
Abstract
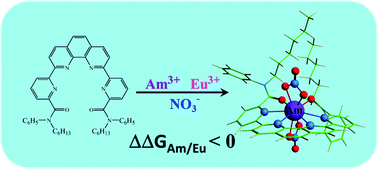
Due to the similar chemical properties of actinides An(III) and lanthanides Ln(III), their separation in spent nuclear fuel reprocessing is extremely challenging. A 1,10-phenanthroline dipicolinamide-based ligand (PhenBHPPA) has been identified to possess a selectivity for Am(III) over Eu(III) and could potentially be used for group actinide extraction. In this study, quasi-relativistic density functional theoretical calculations have been used to disclose the interaction mechanisms of Am(III) and Eu(III) complexes with PhenBHPPA. The electronic structures, bonding nature, QTAIM (Quantum Theory of Atoms in Molecules) analyses and thermodynamic behaviors of the Am(III) and Eu(III) complexes with PhenBHPPA have been explored in detail. According to the Wiberg bond indices (WBIs) and QTAIM analyses, interactions between the ligand and metal cations (Am(III) and Eu(III)) exhibit a weakly covalent character. Thermodynamic analyses show that the charged complexes [ML(NO3)2]+ appear to be the most stable species in the complexation processes. Moreover, it is more energetically favorable for PhenBHPPA to bind to Am(III) compared to Eu(III). Our study could render new insights into understanding the selectivity of the ligand towards minor actinides and the separation of An(III) from Ln(III) via liquid–liquid extraction.

 Please wait while we load your content...
Please wait while we load your content...