Computational prediction of cyclometalated IrIII, RhIII and CoIII amido complexes to capture up to three CO2 molecules†
Abstract
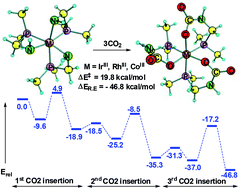
An organometallic complex showing the ability to capture up to three CO2 molecules is unprecedented in chemistry while with convincing theoretical evidence we show that [(CH3)2PCH2NH2] (pcn) based cyclometalated Ir(III), Rh(III) and Co(III) amido complexes can perform such a reaction under mild conditions. The CO2 capture in these complexes occurs via three successive CO2 insertion steps into the M–N(amido) bonds which are driven by modest activation barriers (14.5 to 23.1 kcal mol−1) and highly exothermic products (−18.4 to −47.4 kcal mol−1). The Rh(III) is more effective than Co(III) and Ir(III) and solvation by acetonitrile further enhances the CO2 insertion ability of all three complexes. The feasibility of the successive CO2 insertion reactions has been explained on the basis of the synergistic effect induced by the pcn ligand, viz. the nucleophilic affinity of the nitrogen atom, the ring strain of the [η2-NHCH2P(CH3)2] moiety and the electronic effect of the phosphorous atom.

 Please wait while we load your content...
Please wait while we load your content...