Extremely bulky secondary phosphinoamines as substituents for sterically hindered aminosilanes†
Abstract
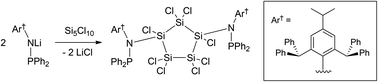
The synthesis of a series of extremely bulky secondary amines with a phosphine function, Ar†(PR2)NH (Ar† = C6H2{C(H)Ph2}2Pri-2,6,4; R = Ph, NEt2, NPri2) is described. Deprotonation with either n-BuLi or KH yields the respective alkali metal amides in some cases. Their reaction with the chlorosilanes SiCl4, HSiCl3, Cl2SiPh2, Cl3Si–SiCl3 and Si5Cl10 allows access to monomeric molecular compounds bearing the extremely bulky amino substituents via salt elimination. The products obtained may serve as precursors for subsequent reduction reactions to access sterically protected low valent and low coordinate silicon compounds.

 Please wait while we load your content...
Please wait while we load your content...