Luminescence properties of some monomeric and dimeric cycloplatinated(ii) complexes containing biphosphine ligands†
Abstract
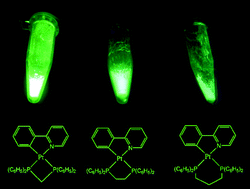
The starting complexes [PtCl(C^N)(dmso)], in which C^N is either ppy = 2-phenylpyridinate, 1a, or bhq = 7,8-benzo[h]quinolinate, 2a, were prepared by a known method using the reaction of [PtCl2(dmso)2] with ppyH or bhqH, respectively, in toluene under reflux conditions. The reaction of [PtCl(C^N)(dmso)], 1a or 2a, with 1 equiv. of a number of biphosphine ligands, P^P, gave the cationic monomeric complexes [Pt(ppy)(P^P)]Cl, for which P^P is either 1,2-bis(diphenylphosphino)ethane (dppe), 3a, 1,3-bis(diphenylphosphino)propane (dppp), 3c, or bis(diphenylphosphino)methane (dppm), 3d; the bhq analogous complex [Pt(bhq)(dppe)]Cl, 4a, was prepared similarly. However, the complex [Pt(ppy)(dfppe)]Cl, 3b, in which dfppe is 1,2-bis(dipentafluorophenylphosphino)ethane, was prepared by the reaction of 1a with excess amount of dfppe. When each of the starting complexes [PtCl(C^N)(dmso)], 1a or 2a, were reacted with 0.5 equiv. of any of the P^P ligands, the dimeric complexes [Pt2Cl2(ppy)2(μ-P^P)], 5a–5d, or [Pt2Cl2(bhq)2(μ-P^P)], 6a–6b, were formed. The complexes were fully characterized using multinuclear (1H and 31P) NMR spectroscopy and elemental analysis. The structures of typical complexes 3a, 3c, 5b, and 5d were also confirmed by X-ray crystallography. The effect of ligands on the luminescent properties of the complexes was investigated and DFT calculations were performed to confirm the assignments.

 Please wait while we load your content...
Please wait while we load your content...